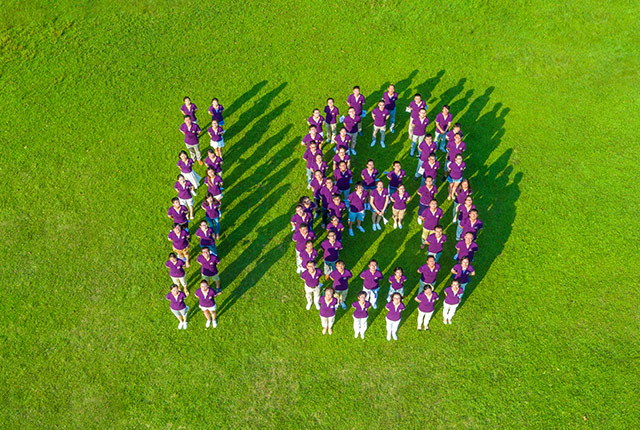Copyright www.hencer.com 苏ICP备11086299号-1
National:025-84563607 International:+86-25-84816997 +86-25-84816812
Add:No.18,jichang Road,Lishui Economic Development Zone,Nanjing,211200 P.R.C Fax:025-57226938 Tel:(025)57212809 57212819 E-mail:hencer@126.com
R&D Overview
Hencer steadfastly upholds the spirit of development, which is “always give prior consideration to technology, put quality of products at a paramount place, lead the industry standard and manage scientifically,” meanwhile being named Research Center of Polysaccharide Iron Compound Medicine in Jiangsu Province and Technology Center of Enterprise Identified by Jiangsu Province. Additionally, Nanjing Lifenergy R&D Co. Ltd, as a wholly owned subsidiary of Nanjing Hencer Pharmaceutical Co. Ltd, annually invests a plenty of money on research and development, which accounts for 8%-10% of sales revenue.
Modern laboratories that conforms to GLP standard are built in R&D center, where is equipped with cutting-edge scientific apparatuses and analytical devices with international caliber. So our R&D center can shoulder a series of medical work, including assessment of project, drug screening, establishments of quality standard of APIs and formulations, clinic research, the process of drug registration and approval, etc.
Hencer orientates its products in nephrology area. There are more than fourteen research projects at present, and six of which are centering on the field of nephrology and three projects have link with the field of cardiovascular and thrombin inhibitors.
Hencer is guided by the concept of "valuable research", sticking to the principle "innovation derived from generics other than purely imitation", targeting at "the best quality in globe" and establishing" patent barriers" and "standard barriers", to constantly enhance the value of products and technology.
2 provincial R & D institutions
14 Independent scientific research project

Total number of 14 core patents
R&D Results
Hencer has 41 licenses of production for APIs, injections, eye drops, oral liquid and oral solid dosage forms. The main products are chemical medicines and others are traditional Chinese medicines.

Up to now, Hencer has 46 valid patents and patent applications, in which there are 13 valid invention patents, 1 American PCT patent and 15 patents applied for invention.

Hencer insists the R&D idea of “innovation derived from generics other than purely imitation” and keeps upgrading the R&D level, to challenge the original products with our highly innovative generic products. After years of efforts, Hencer has gone from being a follower to an improver of product quality standards.

Hencer carries out the research on the original drug development and industrialization around the topic of therapeutic drugs for major diseases and preventing drugs for new type of metabolic syndrome. Hencer also undertakes more than 10 technology projects including state's key new product projects, technological innovation projects of Chinese Small& Medium-sized Enterprises and so on.

R&D Team
Hencer is people-oriented, and the R&D center has a first-class team with professional pharmaceutical background, who are capable with bringing products into industrialization. There are 86 personnel in R&D center, including 2 doctors, 8 senior engineers and 16 intermediate engineers.
At the same time, Hencer employs well-known experts at home and abroad, in order to create technology upgrading in key areas. Hencer's competitive advantages lie in effective combination of talents and resource distributions.