Copyright www.hencer.com 苏ICP备11086299号-1
National:025-84563607 International:+86-25-84816997 +86-25-84816812
Add:No.18,jichang Road,Lishui Economic Development Zone,Nanjing,211200 P.R.C Fax:025-57226938 Tel:(025)57212809 57212819 E-mail:hencer@126.com
SINCE
Nanjing Hencer Pharmaceutical Co., Ltd, established in 1995, is an innovative high-tech enterprise incorporates R&D, manufacture and sales. Hencer has 424 employees, 75% of which have college degree or above. Staffs in every post are professional and experienced. The combination of talent and techniques make Hencer stand out from Chinese pharmaceutical industry.
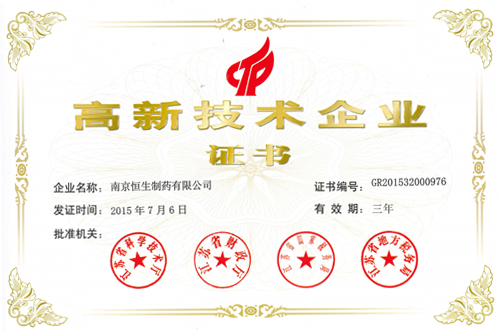
Hencer has been named Research Center of Polysaccharide Iron Compound Medicine in Jiangsu Province, Private Scientific and Technical Enterprise in Jiangsu Province and Credit-Worthy Enterprise in Jiangsu Province.
Nanjing Hencer Pharmaceutical Co. Ltd. steadfastly upholds the spirit of development, which is “always give prior consideration to technology, put quality of products at a paramount place, lead the industry standard and manage scientifically,” on the lead of science, with the core of characteristic products, with knowledge marketing as impetus, to build a leading enterprise focusing on IV iron and branded generic nephrology products.
Hencer’s Manufacturing Center locates in Lishui Economic & Technological Development Zone, Nanjing; the R&D center, namely, Nanjing Lifenergy R&D Co., Ltd, locates in Nanjing Economic & Technical Development Zone; Domestic Marketing Division is located in Changfa Building and International Business Division is located in Golden Eagle Hanzhong Garden.
Hencer and Lifenergy focus on nephrology and cardio-cerebrovascular. Our core products, Iron Sucrose Injection, Ibandronate Sodium Injection and Phloroglucinol Injection, were the first to enter the domestic market and been sold to overseas market.
Compound Earthworm Capsules and Xintongkang Capsules are “Exclusive Protection Varieties of Traditional Chinese Medicine”. Compound Earthworm Capsules is the Hencer’s exclusive product and is the only compound preparation traditional Chinese medicine containing Lumbrukinase being approved by NMPA.
Hencer always targets at the development frontier of high technology. To make the products’ quality and technological level meet the requirements of advanced international standard and become the flagship of Chinese pharmaceutical industry, Hencer keeps enhancing the strength of development, establishing the R&D concept which adapts to global strategy, improving products’ quality and economic efficiency and expanding the export scale.
— 1995 —
CULTURE
Mission:
To improve human health by collecting life energy.
Sprits:
Innovation, Modernization, Perseverance, Responsibility.
Development concepts:
Leading technology, paramount quality, advanced standard,
scientific management.
Vision:
A specialized, globalized and leading
enterprise of IV iron and branded
generic drugs for
nephrology.
2015
2015, Nanjing Hencer Pharmaceutical Co., Ltd assigned 100% equity of Nanjing Lifenergy R&D Co., Ltd and achieved extension of industry chain and integration between R&D and manufacturing.
2007
2007, Ketoacid products started to export. Hencer was determined to become a leading company with specialized and globalized branded nephrology products according to the idea of “developing trade with technology, promoting technology of trade.”
2016
2016, Hencer established Research Center of Polysaccharide Iron Compound Medicine in Jiangsu Province.
2002
HISTORY
2002, Xintongkang Capsules was the first to be approved for the listing and became “Exclusive Protection Varieties of Traditional Chinese Medicine”.
1999
1999, Compound Earthworm Capsules was the first to be approved for the listing and became “Exclusive Protection Varieties of Traditional Chinese Medicine”.
2012
2012, Nanjing Hencer Pharmaceutical Factory renamed Nanjing Hencer Pharmaceutical Co., Ltd. R&D, manufacturing, marketing and management have been upgraded.
1994
Nanjing Pharmaceutical Group merged Lishui Pharmaceutical Factory
2004
2004, Iron Sucrose Injection was approved for the list. It is the first ANDA in China and shortened the gap between China and developed countries in IV iron area.
2004, Phloroglucinol Injection was approved for the list. It is the first ANDA in China and provided treatment with the same quality but lower price to patients who need symptomatic treatment.
2001
2001, Ibandronate Sodium Injection was approved for the list. It is the first approved ANDA in China.
1995
Lishui Pharmaceutical Factory was renamed Nanjing Hencer Pharmaceutical Factory
1972
Lishui Pharmaceutical Factory established.
COOPERATION
Hencer has fully realized the significance of university-industry collaboration, making full use of talented persons from colleges and universities, equipment, information and other advantages to enhance the ability of technological innovation. Hencer also established a stable and long-standing partnership with China Pharmaceutical University, Nanjing University and other well-known domestic universities.
Hencer as well as attaches importance to cooperation with research institutes at home and abroad. For example, Hencer maintains close technical and project cooperation with Nanjing Baijingyu Pharmaceutical Co. Ltd, Nanjing Housheng Pharmaceutical Co. Ltd. and other pharmaceutical companies, to effectively integrate the industry resources for technology innovation and to seek common development.